Table of Contents
The European Chemicals Agency (ECHA) has announced, officially adding two new Substance of Very High Concern (SVHC) on February 4th, 2026. With this inclusion, the Candidate List now contains 253 entries though some are group entries covering multiple chemicals, meaning the actual number of affected substances is higher.
The two newly added substances were identified as meeting the criteria of Article 57 of REACH due to their hazardous properties, including persistence, bioaccumulation, toxicity, carcinogenicity, or other serious risks to human health or the environment. Their inclusion reinforces ECHA’s continued focus on substances that may pose long-term environmental and health impacts and signals increased regulatory scrutiny across affected industries
Here's the updated version based on the latest SVHC Candidate List update:
It includes the addition of two more substances:
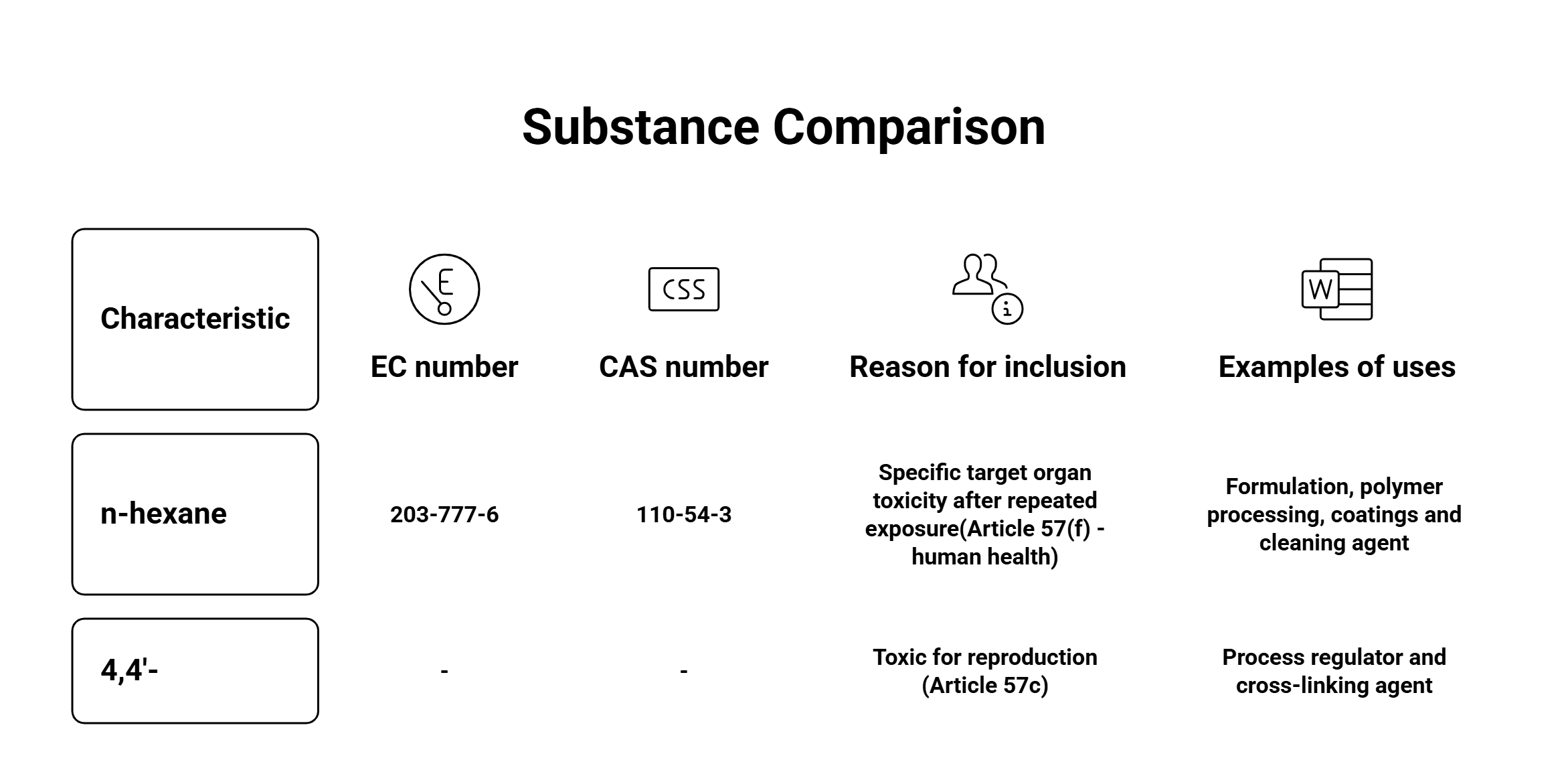
-
n-Hexane (CAS: 110-54-3)
- Reason: Specific target organ toxicity after repeated exposure (Article 57(f))
- Key Uses: Formulation, polymer processing, coatings, cleaning agents.
-
4,4'‑[2,2,2‑Trifluoro‑1‑(trifluoromethyl)ethylidene]diphenol and its salts
- Reason: Toxic for reproduction (Article 57(c))
- Key Uses: Process regulator, cross-linking agent, used in advanced polymers, adhesives, and automotive materials.
If any newly added SVHCs are in your scope, Article 7(2) notifications are due within six months of that date, and Article 33/SCIP obligations continue to apply. The deadline for notifications is August 4, 2026.
For articles containing >0.1% w/w of these substances, you must update Safety Data Sheets, complete SCIP notifications, and disclose via Article 33 as required.
Source: ECHA News.
What Was Added – February 2026 SVHC Update
Date of inclusion: February 4, 2026.
What This Means for You
If any of your articles contain one of the newly added SVHCs above 0.1% weight by weight (w/w per article), the following legal obligations apply under REACH and the Waste Framework Directive:
- Article 33 (REACH): You must provide recipients and, upon request, consumers with sufficient information to allow safe use of the article.
- Article 7(2) (REACH): You must notify ECHA within six months of the inclusion date (by August 4, 2026 for the February 2026 update), unless exposure can be excluded.
- SCIP (Waste Framework Directive): You must submit or update SCIP notifications for affected articles placed on the EU market.
If you manufacture or place substances or mixtures on the market containing these SVHCs, you must:
- Update Safety Data Sheets (SDS) in accordance with REACH requirements.
- Communicate updated hazard and regulatory information down the supply chain.
Failure to comply may result in enforcement action by national competent authorities in EU Member States.
What Happens Next
ECHA and the European Commission will now evaluate the newly added SVHCs for possible inclusion on the Authorisation List (Annex XIV) or future restriction proposals under Annex XVII.
Companies should start preparing now by:
- Assessing whether the newly listed SVHCs are present in any articles, formulations, or components;
- Evaluating technically and economically viable alternatives where applicable;
- Updating internal databases, supplier declarations, and SCIP records;
- Monitoring all regulatory developments related to these substances and their hazard groups.
This update reflects the EU’s continued tightening of chemical oversight and increasing scrutiny of substances with persistent, bioaccumulative, toxic, carcinogenic, mutagenic, or endocrine-disrupting properties.
How to Stay Compliant
Here’s what your compliance team should prioritize right now:
- Screen your BOMs and supplier declarations for the newly added SVHCs.
- Request updated documentation (FMDs, SDS, IPC-1752A, or IEC 62474 declarations).
- Submit Article 7(2) notifications to ECHA within six months of inclusion (by August 4, 2026, if applicable).
- Update SCIP submissions for affected articles placed on the EU market.
- Communicate SVHC information clearly throughout your supply chain in line with Article 33 requirements.
Related REACH Resources
- Understanding REACH Lists: Candidate vs Annex XIV vs XVII
- SCIP Database Notification: Requirements & Process
- IPC-1752A Material Declaration Standard
- EU Waste Framework Directive & SCIP Database
How Acquis Helps with REACH Compliance
Acquis Compliance provides automated solutions to help you stay ahead of REACH and SVHC obligations:
- Automated Compliance Tracking – Real-time SVHC monitoring and update alerts.
- SCIP Reporting Automation – Streamline notifications with pre-validated data.
- Supplier Declaration Management – Request, verify, and centralize supplier documents.
- Bill of Materials (BOM) Analysis – Detect affected parts and components instantly.
- Regulatory Intelligence & Alerts – Get notified when your substances are added or restricted.
The Final Word
With the February 4, 2026 update, the REACH Candidate List now contains 253 entries, and the regulatory direction is clear — the EU continues to expand oversight of substances of very high concern.
If any of the newly added SVHCs are present in your products or supply chain, now is the time to assess, document, and strengthen your compliance controls before enforcement pressure increases.
Book a Demo with Acquis Compliance Stay compliant and confident with automated REACH and SVHC tracking. Book a demo or contact our team to learn how Acquis can streamline your REACH management.
